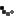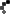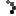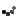DermaRite Industries is recalling nearly four dozen lots of its hand soaps and lotions nationwide after discovering contamination with Burkholderia cepacia (B. cepacia), a bacteria that can cause life-threatening sepsis in vulnerable individuals. The affected products were distributed across the U.S. and Puerto Rico.
The recall covers four products:
- DermaKleen – Antiseptic lotion soap with Vitamin E for reducing bacteria on the skin.
- DermaSarra – External analgesic for temporary relief of itching from dry skin, insect bites, detergents, or sunburn.
- KleenFoam – Antimicrobial foam soap with Aloe Vera for handwashing after diaper changes, assisting sick individuals, or before caring for someone under medical treatment.
- PeriGiene – Antiseptic cleanser for the perineal area.
Consumers are urged to check lot numbers and expiration dates to determine if their products are part of the recall.
While B. cepacia is commonly found in soil and water and poses minimal risk to healthy people, it can cause severe infections in immunocompromised individuals. If the bacteria enter the bloodstream through a cut or wound, it can trigger sepsis — a dangerous, body-wide inflammatory response that can be fatal without prompt treatment.
The U.S. Centers for Disease Control and Prevention (CDC) notes that symptoms range from no signs at all to severe respiratory infections, particularly in people with cystic fibrosis or chronic lung disease. Early symptoms can include fever, fatigue, and localized infection at the entry point.
So far, DermaRite reports no known illnesses connected to the recalled products. The company has notified distributors and customers to destroy affected inventory and advises anyone who may have used the products and developed symptoms to contact a healthcare provider immediately.
Full details, including lot numbers and expiration dates, are available on DermaRite’s official recall notice.